- Posted in: White Papers & Case Studies
- By ProMed Staff
INTRODUCTION
Wearable technology made its debut in the mainstream population in the late 70’s with a wearable calculator that mimicked a wristwatch. Since then, the technology behind electronic devices worn on the body has expanded into a wide range of applications including smart watches that monitor heart rates, virtual reality headsets for entertainment, sensing devices to measure blood-glucose levels, and skin patches with sensors that can transmit a wearer’s vitals wirelessly. Within the medical device industry, wearable devices, or wearables, are a growing segment due to the amount of real time data they can collect and supply. Many aspects of this technology have been adopted into medical devices to aid in patient monitoring and compliance, or immediate communication between patient and practitioner. In the latest wave of innovation, device manufacturers are beginning to look for ways to deliver active therapy through wearable devices.
ProMed has partnered with several innovative medical OEMs to address the challenges of developing wearable devices that deliver heat or electric therapies to the patient by using electrically conductive (EC) silicone elastomers. From mouthguards to treat periodontitis in a non-surgical manner to wristbands that reduce tremors associated with Parkinson’s disease (Figure 1), ProMed has developed a custom manufacturing approach for each application to successfully reach the commercial phase of their device. This article will take a look at the approach taken and challenges that were overcome in the development cycle.

Figure 1: EC Silicone Wearables molded by ProMed. Left: Biolectrics OraflowTM device. Right: Cala Health klQTM device.
BACKGROUND
Electrically conductive silicones are a novel material which combines the stability, biocompatibility, and patient comfort of a silicone elastomer with increased conductivity from conductive additives. The challenge of working with EC silicones lies in selecting the right combination and balance of silicone formulation, conductive additive type and loading level, and molding method and tool design to produce a component that meets the requirements of the application from a mechanical and conductive standpoint (Figure 2).
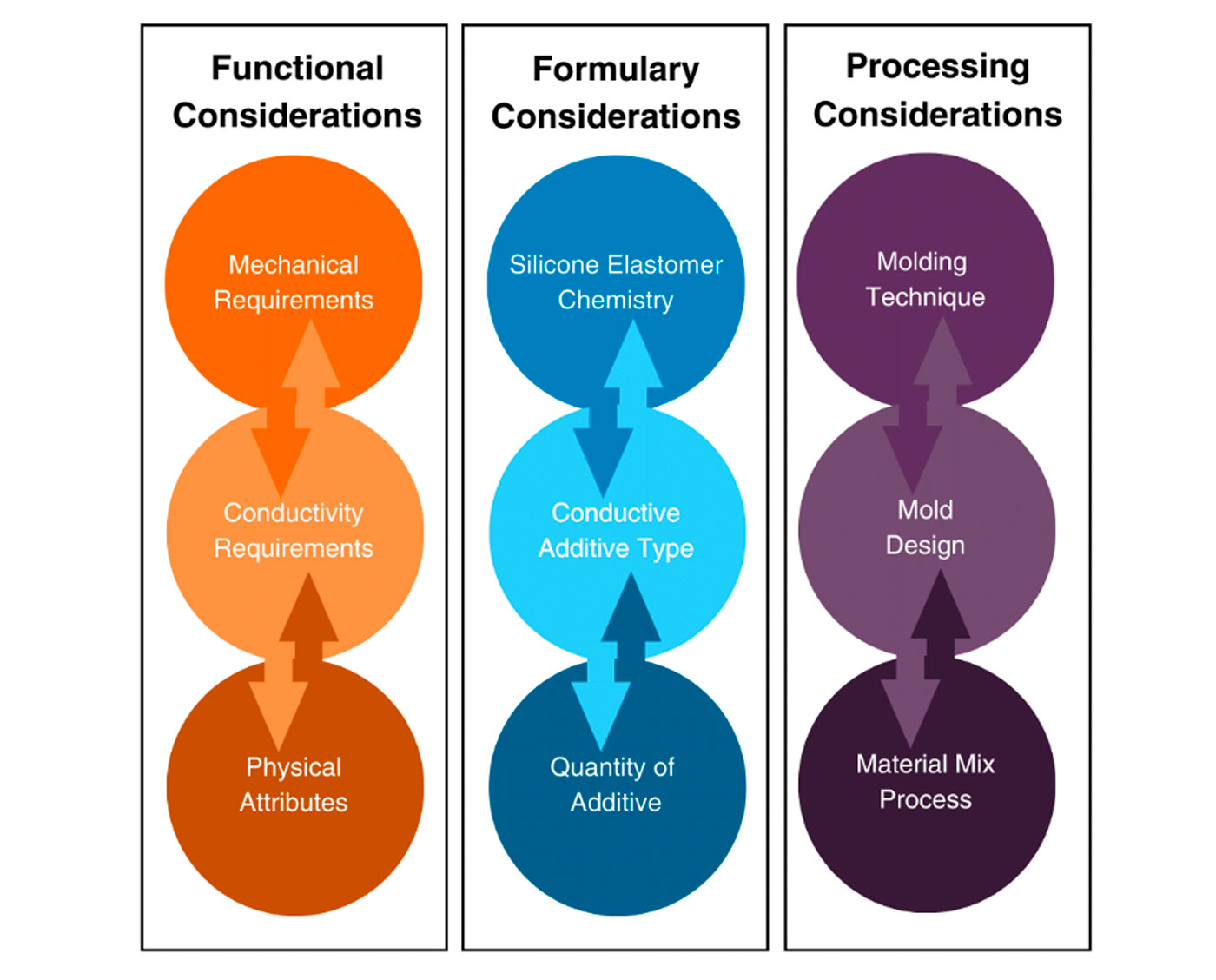
Figure 2 EC Molding Considerations
Silicone Formula Selection
There are three common silicone elastomer formulations used in medical molding, high consistency rubber (HCR), liquid silicone rubber (LSR), and room temperature vulcanizing (RTV) materials. HCR materials are a high viscosity formulation that is typically compression or transfer molded. LSR and RTV formulations have lower viscosities that are commonly injection molded. The initial selection of a base silicone material for an application is driven by application requirements such as strength, flexibility, or hardness needed for the device to perform or feel as the designer intends. The molding style dictated by the formulation of silicone elastomer will have a significant impact on the manufacturing method adopted when working with an EC silicone.
Conductive Additive Selection and Loading Level
Silicone elastomers on their own are insulative and require a critical concentration, or percolation threshold, to exhibit electrically or thermally conductive characteristics. At the percolation threshold there are sufficient additive-to-additive connections within the silicone matrix to transmit heat or electrical charge. To ensure the conductive performance is uniform across a molded silicone component, it’s critical to have homogenous additive dispersion and proper additive-silicone adhesion. Carbon fiber (CF) and carbon nanotubes (CNT) are proven additive choices for modifying the electrical properties of silicone, though CNTs require a lower weight percentage (wt%) added to reach the percolation threshold. The wt% required to achieve the necessary conductive performance is important as the mechanical attributes of the silicone will degrade as the wt% of additive is increased.
Molding Method and Tool Design
Between injection and compression molding processes, silicone elastomers may experience very different forces and temperatures which impact the conductive performance of an EC silicone. Specifically, the shear force imparted onto the elastomer as it is injected into the mold directly correlates with the percolation threshold for the conductive additive of choice. As the shear force increases, so will the percolation threshold and in turn the surface resistivity of the molded elastomer will also increase. Understanding how to minimize the shear effects through mold design and process optimization is critical to the development of a conductive silicone component. Between injection and compression molding processes, there is a greater amount of shear force introduced in an injection process, whereas compression molding yields a very isotropic product due to the lower shear required. Though compression molding produces more consistent product, the process is not favorable for over-molding applications. In such applications, it’s necessary to mitigate the shear force of an injection molding process through the tool design which may include adjustments to the style or size of gate used.
To assess the impacts of the three primary factors in molding with EC silicones, ProMed designed and injection molded 4 styles of silicone pad to test resistivity and resistivity uniformity across elastomer type, additive type, and gate style. Gate styles assessed were a Tab Gate with rectangular cross-section and narrow diameter of 0.009”, and Body Gate with circular cross section and diameter of 0.040” as shown in Figure 3. The sample sets outlined in Table 1 were made and tested in triplicate.
Table 1: Sample Preparation
| Silicone Elastomer | Conductive Additive | Gate Style | |
| Sample A | RTV- 60shA | CF | Tab |
| Sample B | LSR- 65shA | CNT | Tab |
| Sample C | RTV- 60shA | CF | Body |
| Sample D | LSR- 65shA | CNT | Body |
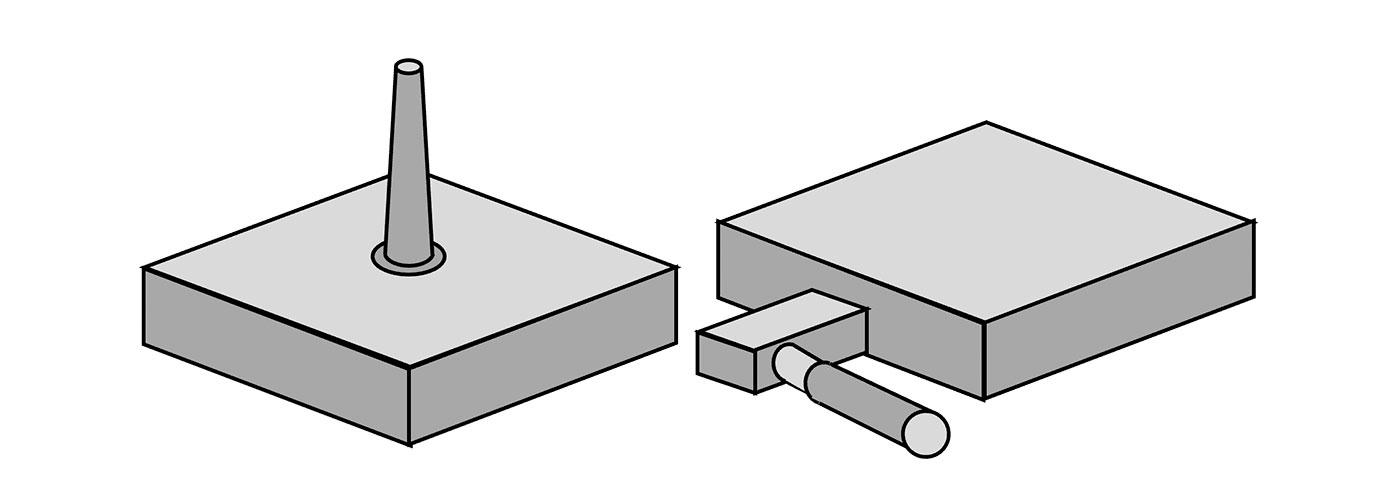
Figure 3 Gate Orientation for Molding Study. Left Direct body gate Right Tab Gate
As indicated in Figure 4, the average measured sheet resistivities for LSR with CNT added are very consistent and within a targeted range of 20-100 Ω/Square for both gate styles. A slight increase in resistivity is seen in the tab gated sample which is attributed to the increased shear force exerted though the more constricted gate diameter. Samples made with RTV elastomer and a CF additive exhibited greater variability in resistivity for both gate designs, with a significantly wider spread with the tab gated sample. Similar to the LSR based samples, the resistivity rose in the tab gated RTV sample due to the shear force associated with the gate style. Because the CF additive has a larger particle size than CNTs, the influence of gate design is much greater.
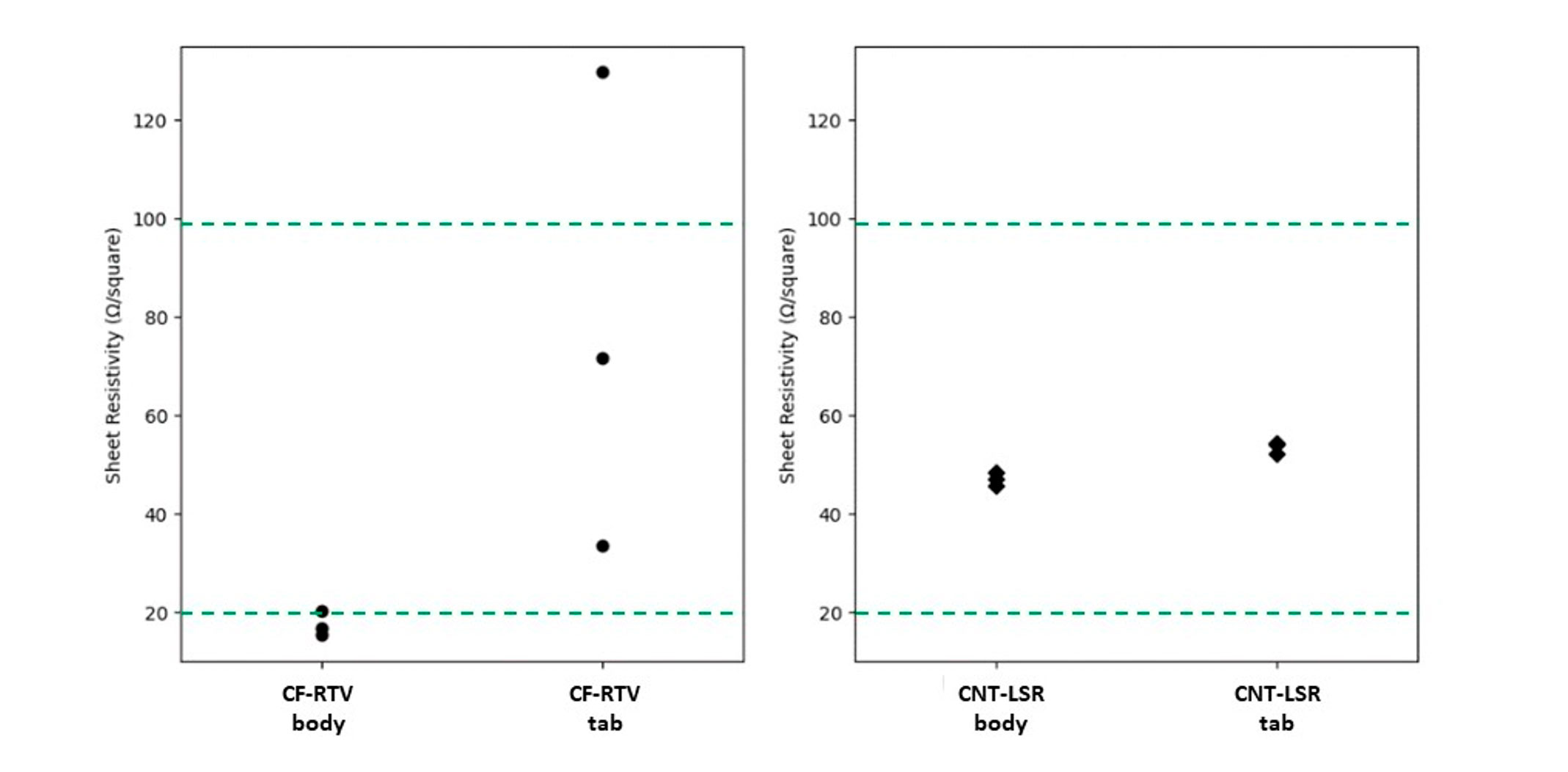
Figure 4: Sheet resistivity measurements for injection molded EC silicones.
Sheet resistivity was measured from three samples injection molded using CF-RTV and CNT-LSR silicones. In both cases, identical samples were molded using tab and body gates. Gate dimensions were consistent with typical designs for production molds. The target resistivity range for typical skin applications is indicated by the dashed lines.
The effect of the gate on surface resistivity is more clearly understood through sheet resistivity maps shown in Figure 5. Note, the false color scale is logarithmic and for tab gated samples, the gate would be located at the left hand side of the map. The consistent averages for the LSR with CNT additive samples observed in Figure 4 are reflected in the measured uniformity from the surface maps. The variability in the average resistivity for the RTV with CF additive samples is better understood considering the increased local resistivity along the left side of map A which correlates to the tab gate location. The impact of gate design and size is significantly more pronounced when working with the larger CF additive in an EC silicone elastomer.
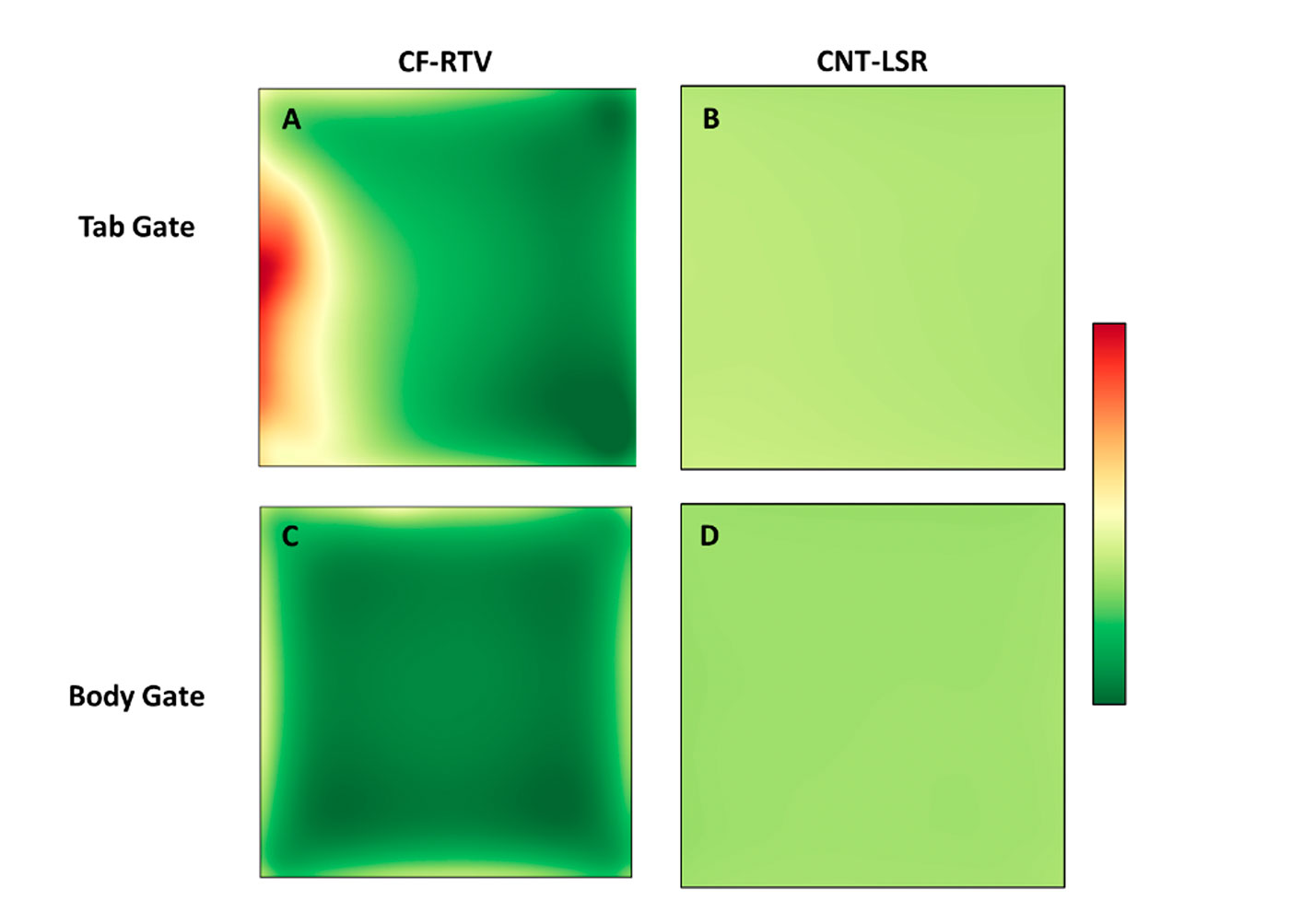
Figure 5: Sheet resistivity maps for CF-RTV (left) and CNT-LSR (right) injection molded samples.
CONCLUSION
Understanding the impact and interaction of elastomer selection, conductive additive material selection, and molding process parameters is critical to manufacturing with EC silicone materials for wearable device applications. It is crucial for the end device designer and manufacturer to work closely together to performance requirements and potential constraints to determine the best path forward to develop consistent conductive componentry. In order to avoid costly mistakes or project delays, taking an iterative approach in the early stages of a project is recommended to find the right combination for success in a specific therapeutic device.
