ProMed has provided contract manufacturing services supporting long term implantable components and assemblies for over three decades. Whether the application be lifesaving or life enhancing, our commitment to supporting the long-term implantable device industry has never wavered.
Silicone elastomers have a long history of use in implantable device applications, with the first being a hydrocephalus shunt in the 1950’s. Since then, it is arguably one of the most researched biomaterials to date and has enabled numerous device designs to become approved for use supporting a wide variety of therapies. Today, implant grade high purity plastics such as PEEK, polycarbonate and polysulfone are available for use as viable biomaterial candidates.
ProMed has extensive experience serving customers with devices in the following implantable spaces:
- Cardiovascular: Pacing and defibrillation devices including suture sleeves, seals, connector boots, headers, desiccant pads, lead components and tines
- Ophthalmology: Intraocular lenses, retinal detachment devices, punctal plugs and ocular implants
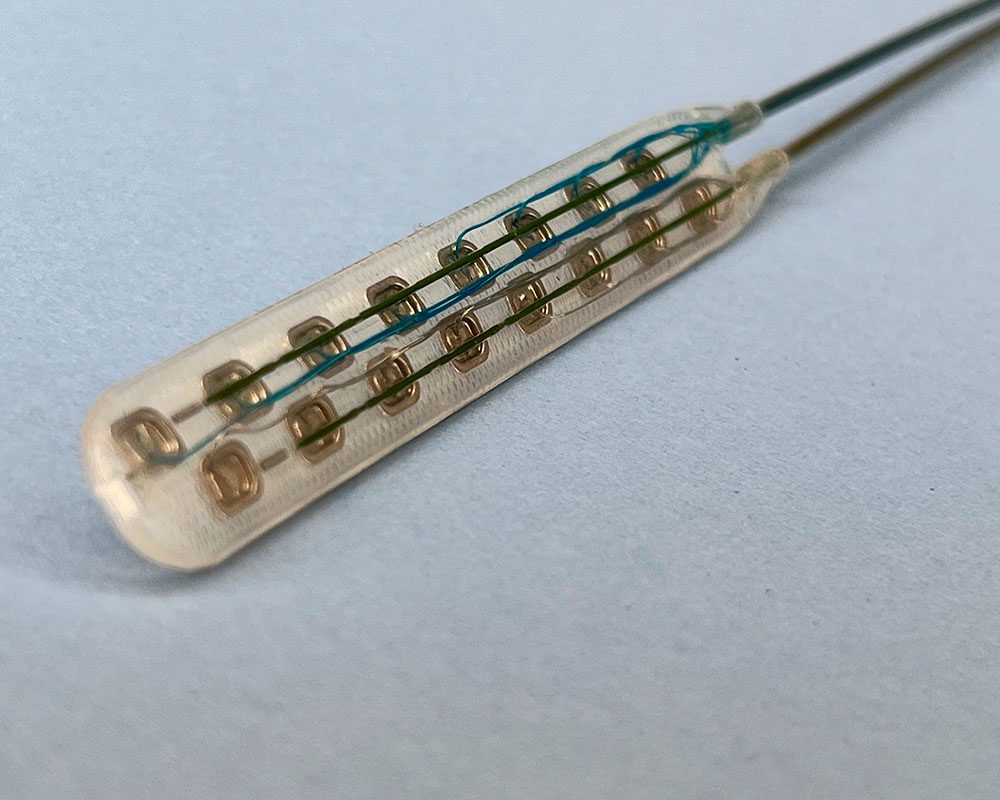
- Neurological: Seals, connector boots, strain reliefs, anchors, headers, paddle lead assemblies
- Orthopedic: Toe and finger joints, spacers, bone and joint implants
- Diabetes: Infusion ports and pumps, balloons, shunts, catheters and valves, implantable glucose monitor and sensing devices
- Auditory: Components for cochlear implants and hearing-impaired devices